How to Determine Cathode and Anode in Half-reactions
The zinc loses two electrons to form Zn 2. Test yourself solution link-httpsyoutubeVPHUzf-_qc0.
E 034V - -076V 110V.
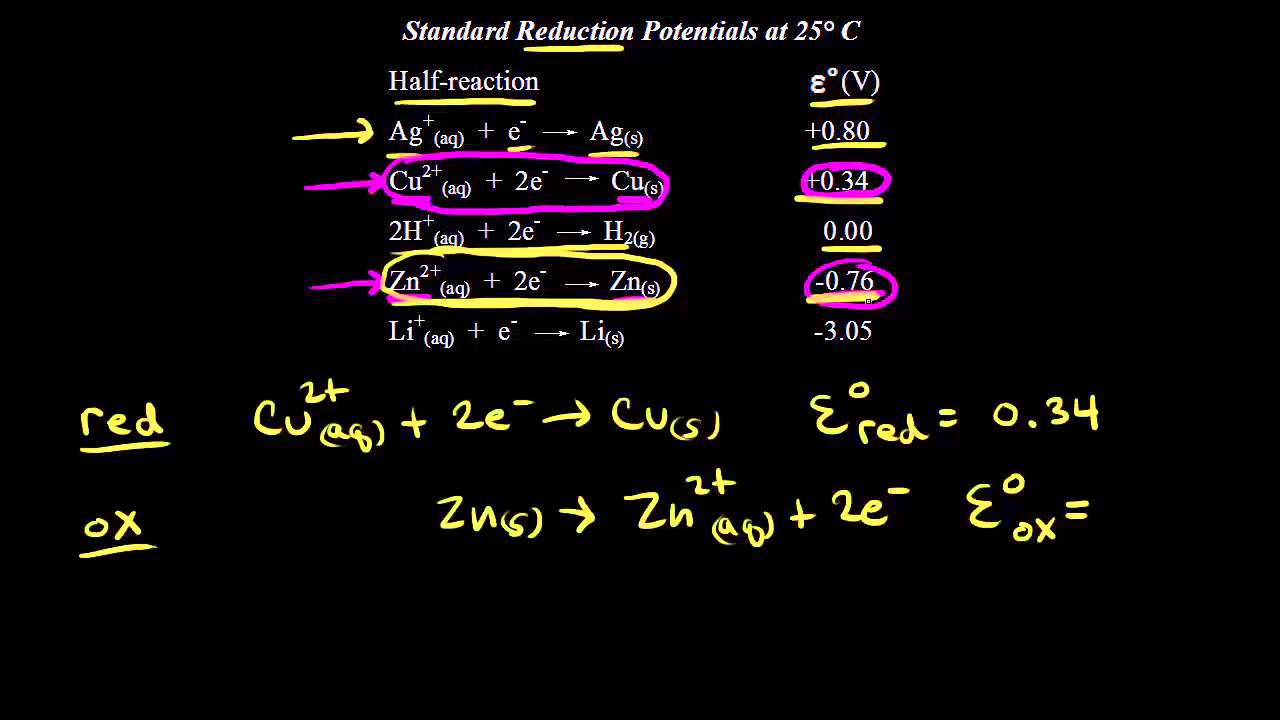
. It acts as an electron acceptor. When you are given two half reactions and their respective potentials have Ecathode- Eanode result in a positive E. E o cell E o cathode -.
In an electrolytic cell oxidation reaction takes place at the anode. Zn s Cu 2 aq Zn 2 aq Cu s It is possible to look at the half-reaction taking place in a half-cell and determine which electrode is the anode and which is the cathode. When given two half.
The following two half-reactions take place in an electrolytic cell with an iron anode and a chromium cathode. 2 C102g Pbs 2 C102 aq Pb2aq 7. Determine which half-reaction occurs at cathode and which occurs at anode and calculate Ecell for the following overall cell reactions use Table 191 in the texbook.
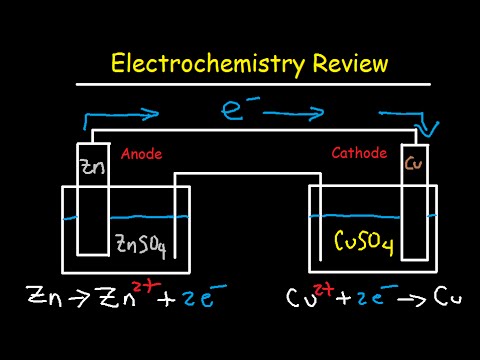
Whereas the cathode is represented by the right-half of the cell diagram and demonstrates the reduction half-reaction. The anode is usually the positive side. One of them is zinc and the other one is copper.
LEO Lose Electrons Oxidation. Cu2aq 2e- Cus E 034V Zn2aq 2e- Zns E-076V You would want to make Zn2Zn become the anode to maintain a positive E. Oxidation is loss at the anode therefore the oxidation half-reaction occurs in the half-cell containing the anode.
Remember LEO the lion goes GER. In order to determine which electrode is the anode and which is the cathode the two half-reactions must be considered. Calculate the value of cell potential for the half-reaction CuI e- Cu I- I find the other half reaction is Cu e- Cu E052V Homework Equations I use the Nernst equation to solve for the standard cell potential then fill in E Ecathode - Eanode to solve the remaining unknown.
The anode is the electrode where electricity moves into. A half-reaction is the net reaction that occurs with the species being oxidized or reduced and shows the flow of electrons. Take the two half-reactions in the Cu-Zn cell.
Trick to identify Anode and Cathode in a cell reaction. Looking closely the anode is represented by the left-half of the cell diagram and demonstrates the oxidation half-reaction. For example if given the half reactions.
Solution for 6 Write the anode and cathode half reactions and calculate Ecell for the following redox reaction. On a commercial battery the anode and cathode are clearly marked - for anode and for cathode. Standard Reduction Potential Table.
Video made by Kathryn-Marie Cailing. Using copper as cathode the cathode reaction is. At the anode is oxidation where the chemicals lose electrons and at the cathode is the reduction where the chemicals gain electrons.
The solubility product for CuIs is 11 x 10-12. Sometimes only the terminal is marked. Zn s Cu 2 aq Zn 2 aq Cu s It is possible to look at the half-reaction taking place in a half-cell and determine which electrode is the anode and which is the cathode.
GER Gain Electrons Reduction. Ni2l 2e Nis in the terminal the cathode. The cathode is the electrode where electricity is given out or flows out of.
Write the reduction half-reactions that turn. A cathode is a negative side. Cr 3 aq 3e- àฏ Cr s During the process the mass of the iron anode decreases by 175 g a.
Determine the half reactions occurring at the cathode and anode in the electrolysis of aqueous Na2SO4 and write the overall reaction occurring in the electrolysis cell. In the terminal the anode. Cell notation is a simplified way to.
If youre setting up a galvanic cell youll need to keep the redox reaction in mind to identify the electrodes. If one element A is above B in the standard electrode potential series then the one with greater oxidising power or reducing agentieB is anode. Overall what weve found can be summarized.
Sns 2Cu²aq Sn²aq 2Cuaq. Cu2_aq2e-rightarrow Cu_s Cell Notation. The half-reaction on the anode where oxidation occurs is Zn s Zn 2 aq 2e.
Cu s Cu 2 a q 2 e. It acts as an electron donor. Electrons always flow through the external circuit from A to C.
Here the copper ions gain electrons and become solid copper. Determine what ions came from the compound. Fe s àฏ Fe 2 aq 2e- Reduction.
While the one with greater reducing power or oxidising agent ie A is cathode. Find the equilibrium constant for the reaction Crs Zn2aqCr2aq Zns if the standard cell emf is 076 V at the cathode and 091 V at the anode. Cr2O²aq 14 H aq 6 I aq 2 Cr3 aq 3 12s 7 H2O1 b.
Zn s Cu 2 aq 2e Zn 2 aq Cu s 2e or if we cancel the electrons. On a battery the bumpy side is and the smooth side is -. The half-reaction on the cathode where reduction occurs is Cu 2 aq 2e Cu s.
Subtract the electrode potential of the anode from that of the cathode and you get the electrode potential of the cell or voltage. To adjust the PH you are adding NaOH and HCl how does this affect the reaction.
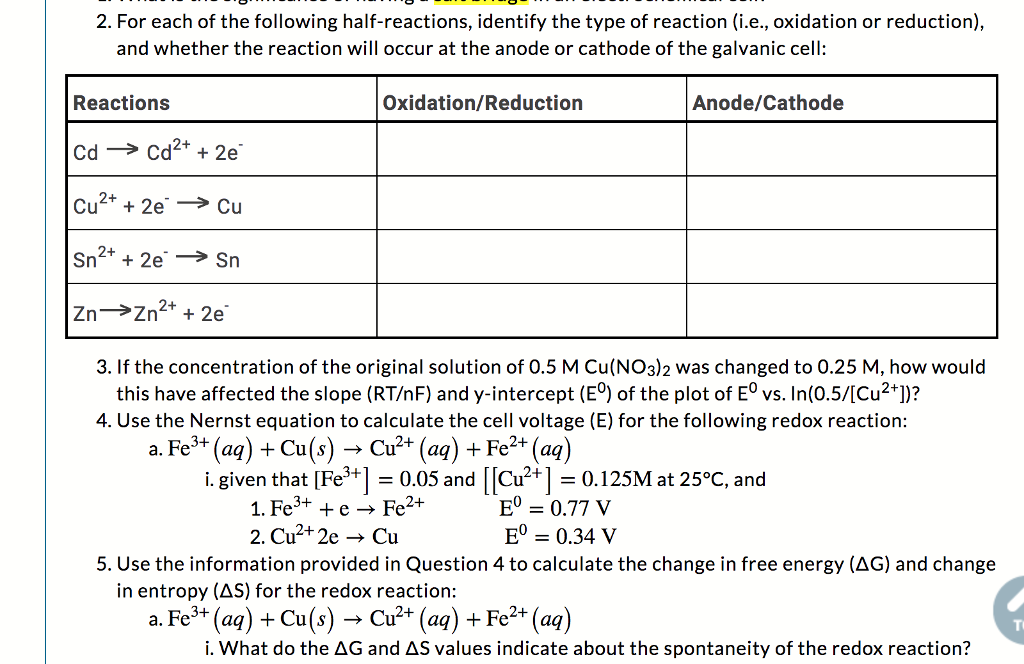
Solved 2 For Each Of The Following Half Reactions Identify Chegg Com

Voltaic Cells Galvanic Cells Electrochemical Cells Chemtalk

Electrochemistry How Do You Tell Which Is Cathode And Which Is Anode Given Half Reactions And Ered How Can You Tell If It S Galvanic Or Electrolytic R Mcat

Trick To Identify Anode And Cathode In A Cell Reaction Youtube

Cathode Vs Anode Half Cell Reactions How To Determine Cathode Anode Video Lesson Transcript Study Com
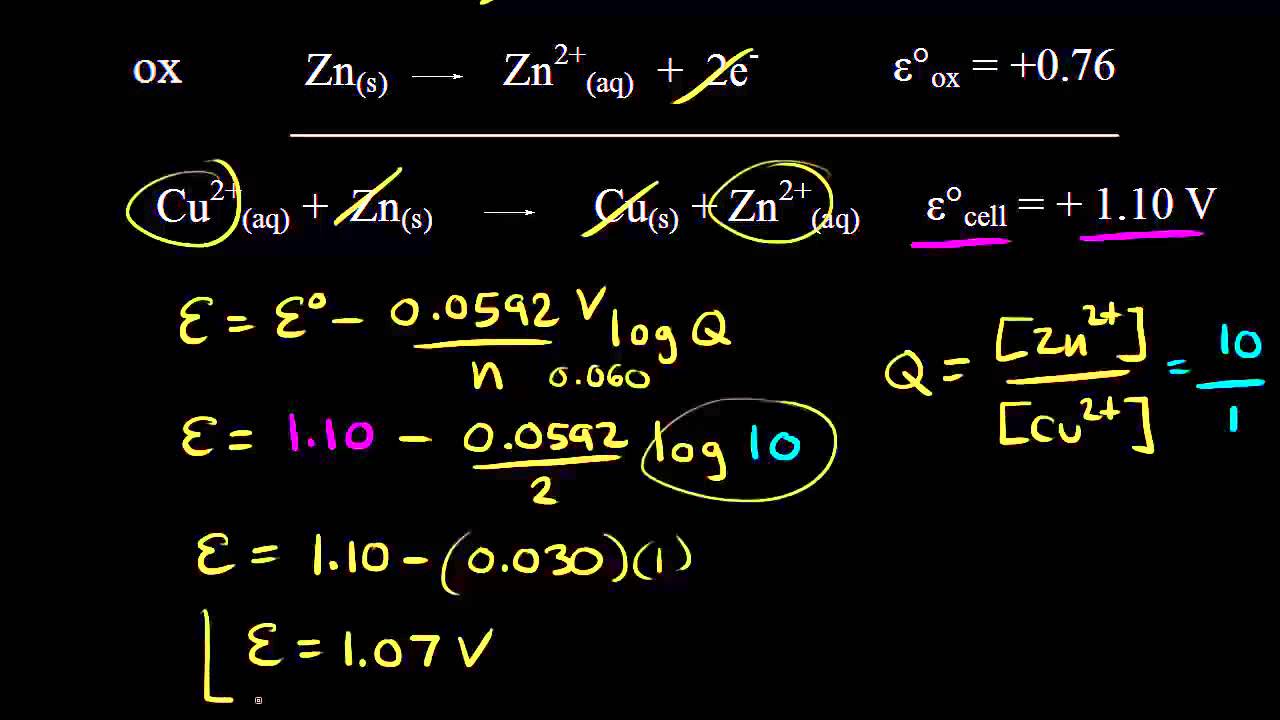
Using The Nernst Equation Video Khan Academy

Standard Reduction Potentials Video Khan Academy

Chemical Kinetics Class 12 Notes Vidyakul Chemical Kinetics Chemistry Class Chemical

Question Video Calculating The Standard Cell Potential For A Gold Nickel Cell Nagwa

Electrochemistry Voltaic Cell Or Galvanic Cell The Energy Released In A Spontaneous Redox Reaction Can Be Used To Perform Electrical Work A Voltaic Ppt Download
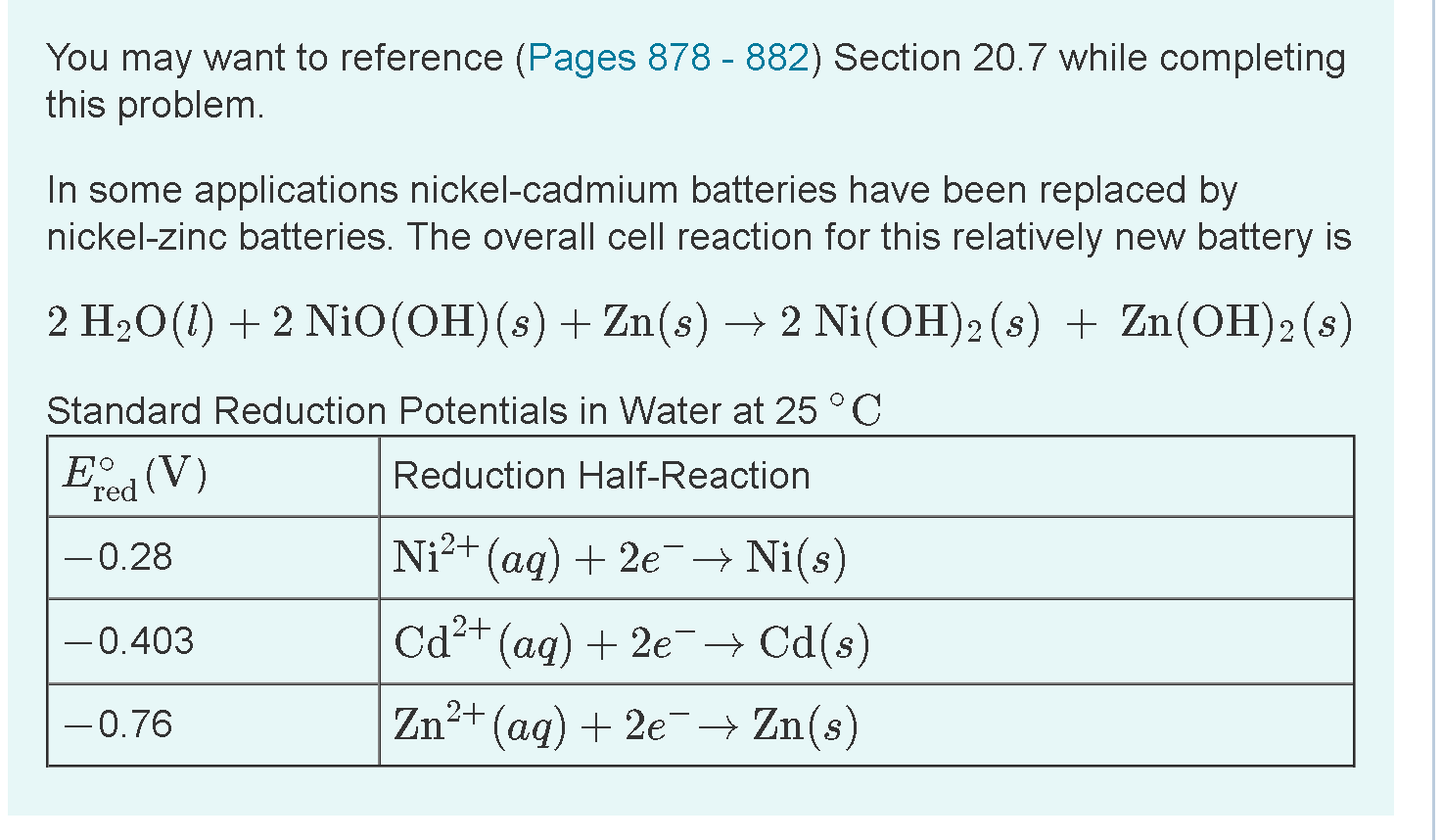
Solved What Is The Cathode Half Reaction What Is The Anode Chegg Com

Galvanic Cells Standard Reduction Potential Ecell Handout And Worksheet Chemistry Worksheets Galvanic Cell Reduction Potential
Electrochemistry Review Cell Potential Notation Redox Half Reactions Nernst Equation Youtube

Describing Redox Reactions Handout And Worksheet Redox Reactions Handouts Science Chemistry
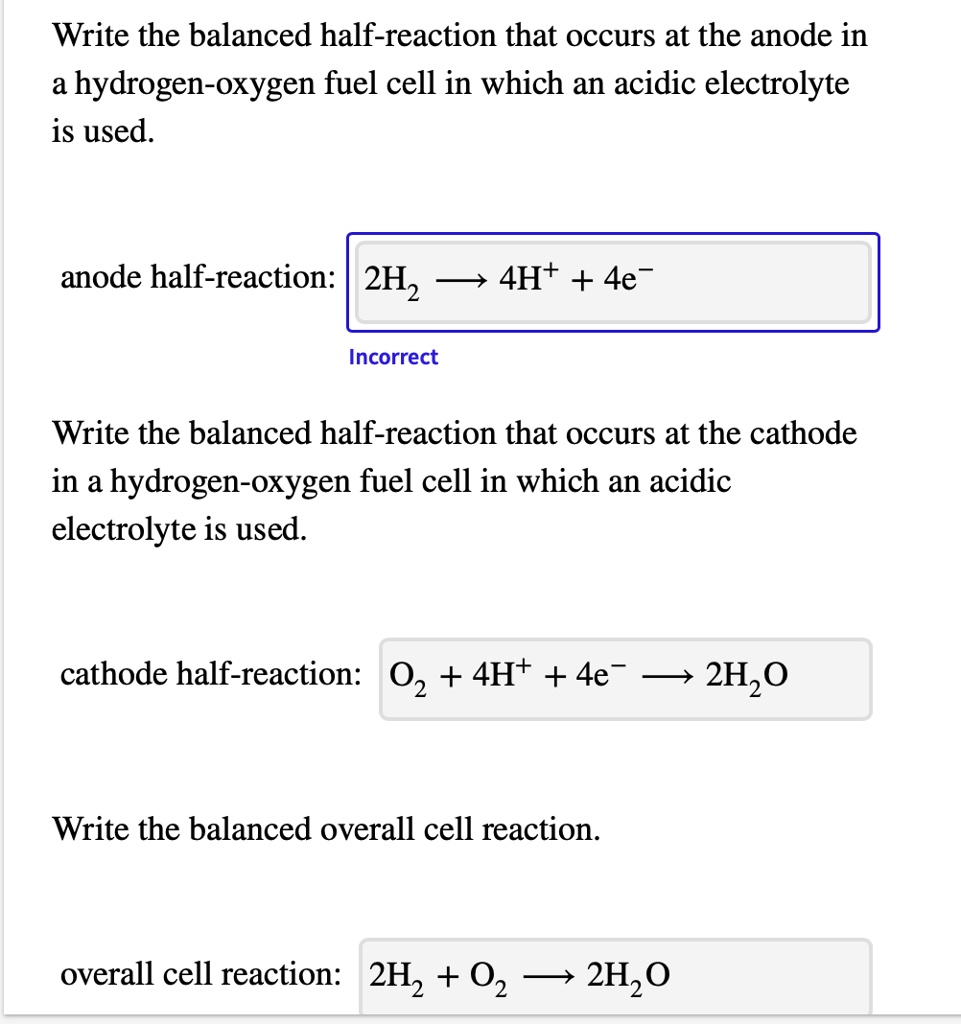
Solved Write The Balanced Half Reaction That Occurs At The Anode In A Hydrogen Oxygen Fuel Cell In Which An Acidic Electrolyte Is Used Anode Half Reaction 2h2 4h 4e Incorrect Write The Balanced Half Reaction




Comments
Post a Comment